Gold: Au
atomic number: 79
atomic mass number: 196.96655
subatomic particles that are equal in number are: 118 neutrons, 118 electrons.
Model made from Fruit Loop cereal. Yellow= Protons; Orange= Neutron; Blue and Purple= Electrons.
atomic number: 3
atomic mass number: 6.941
subatomic particles that are equal in number are: 3 protons, 3 electrons.
Model made from Tinker Toys. Red= Protons; Yellow= Neutrons; Short, hollow dowels= Electrons.
Titanium: Ti
atomic number: 22
atomic mass number: 47.867
subatomic particles that are equal in number are: 22 protons, 22 electrons.
Model made from play dough connected by toothpicks and uncooked spaghetti.
4. I would make an isotope for the model gold by: Adding the same number of protons, but different number of neutrons (p.64).
There are 21 isotopes of gold. Half-life of Au-194 is 1.6 days. Half-life is the time required for half of the atoms of radio isotopes to undergo decay. What we referred to as gold is Au-197, a stable isotope. It is inert in nature. It is neither corroded by air nor affected by most of the reagents. http://www.buzzle.com/articles/gold-the-element.html
One radioactive isotope of gold is commonly used to treat cancer. http://www.chemistryexplained.com/elements/C-K/Gold.html
A gold iisotope has the same atomic number as the element gold itself, but have different mass numbers (same number of protons, but different number of neutrons).
5. Considering the overall volume of my element models the nucleus (protons and neutrons) makes up most of the volume of the atom.
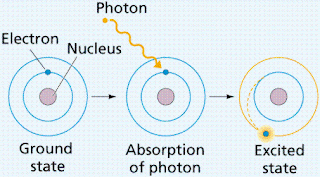
6. Here, my model of gold (Au) is shown with another image when energy excites an electron: Electrons in the lowest energy state are referred to as being in the ground state; when energy is absorbed by electrons they then become excited then emits a photon of energy when returning to its lower state.
8. Some elements differ in colors when they are excited due to igniting different energy levels they raise and fall to.
9. With the Fourth of July coming up- the following explains how the colors of fireworks arise:
Atoms are broken apart; a reaction occurs which happens when the rearrangement of atoms happens (slide 7). Fireworks are attributive to specific elements. For example, red is produced by strontium compounds, whereas barium compounds are used to produce green. Another example is when white light from an incandescent lamp, for instance, is passed through a prism; it produces a continuous spectrum of rainbow of colors (Hill & Kolb p. 61).
10. Explain the overall organizational structure of the periodic table.
The table is arranged with the elements in order of increasing atomic mass (rows/period); however, in a few cases a heaver element comes before a lighter one in order to place elements with similar chemical properties in the same column (family/group); some resemble chemical properties. Gaps were purposefully left blank for elements yet undiscovered.
11. List two example elements for each of these groups or classes: Alkali Metals (Hhydrogen, Lithium), Alkaline Earth (Beryllium, Magnesium), Halogens (Francium, Radium), Noble Gases (Helium, Neon), Transition Metals (Titanium, Gold), Non-Metals (Arsenic, Oxygen), and Metalloids (Arsenic, Iodine).



No comments:
Post a Comment